GOOD Manufacturing Practice (GMP)
INTRO GMP
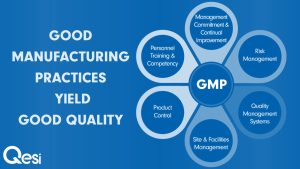
The full term of GMP is Good Manufacturing Practice. GMP certification is a system which design to ensure the products are consistently produced follow the quality standards. Therefore, manufacturers, processors, and packagers are encouraging proactively adopt this system as a result ensure their products are safe and pure. Besides, GMP regulations require a quality approach to manufacturing, enabling companies to minimize or eliminate instances of contamination, mix-up, and errors.

Good Manufacturing Practice GMP guidelines are applied to:
- Hygiene
- Protection of environment
- Control of manufacturing process
- Distribution methods
- Control of procedures and record-keeping
- Procedures to follow when there is a recall or investigation of defects
Who is GMP for?
GMP is suitable for food manufacturers, food processors, and packagers, beverage manufacturer, feeds manufacturer, pharmaceutical, etc.
Benefit for GMP:
- Increase food safety, prevent contamination
- Reduce product defect
- With GMP implementation, can give more confident to the buyer
- More competitive in the market
- Improve brand image
- Better comply to legal regulation
